Applying for a Biospecimen Ancillary Study
Add Health maintains a repository of biospecimens that were collected at various timepoints during the study that are available for current use in research. For more information about the use and storage of biospecimens in Add Health, please review the Biospecimen Reserve Policy.
Phase 1 – Getting Started
To gain a more detailed understanding of the application and study process, please refer to the Biospecimen Ancillary Study Researcher Guidelines. Before applying, Biospecimen Ancillary Study investigators should:
- Determine whether their assay can be conducted in the Add Health laboratory or will need to be performed by another laboratory.
- Determine the smallest possible biospecimen volumes needed to complete their proposed work.
- Only request those minimum volumes.
- Only run samples in singleton, when appropriate.
When planning ancillary studies involving biospecimens, Add Health recommends and highly prefers that all testing and long-term storage of biospecimens be conducted at the Laboratory for Clinical Biochemistry Research (LCBR), our partner laboratory at the University of Vermont (UVM). Our Add Health Biology Team has a longstanding working relationship with LCBR, all of our venous blood biospecimens are archived and monitored there, and we can best implement all aspects of your biospecimen testing at this laboratory.
If your ancillary study requires a specialized laboratory outside of LCBR, please provide strong written justification for this request that includes plans for final disposition of any leftover sample. In addition, when preparing your budget please include $7.50 per biospecimen to cover the cost of pulling and shipping blood from LCBR to your approved outside laboratory.
Biospecimen Availability
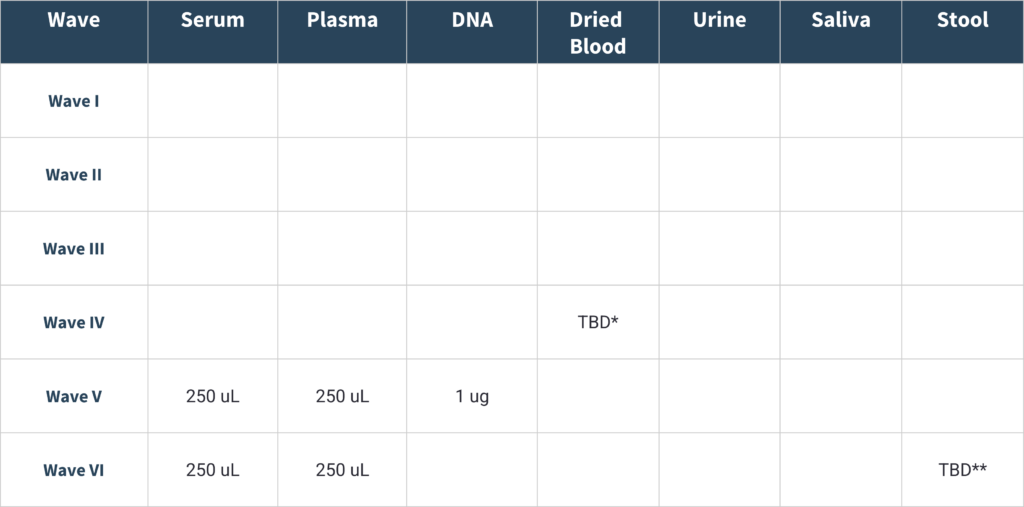
*Available beginning fall 2025-requires additional fees for lab pulling and processing. Allowable volume TBD
**May be limited sample size-requires additional fees for lab processing. Allowable volume TBD
Phase 2 – Applying for a Biospecimen Ancillary Study
1. Submit a brief 1-page Concept Proposal to addhealth_ancillary@unc.edu for Add Health review. Examples are available under the Documents and Resources tab.
2. Address feedback and resolve issues from the preliminary concept proposal review.
3. Submit a Biospecimen Ancillary Study Application Form for Add Health review. Examples are available under the Documents and Resources tab. Please include the number of variables and a narrative description (12 pages maximum) consisting of:
- Why Add Health?
- Specific Aims
- Brief background and significance
- Conceptual framework and hypotheses
- Data and/or biological materials requested or to be collected
- Sample size and justification (i.e., formal power calculation)
- Analysis Plan for each aim
- Study timeline
4. Receive Add Health review. The outcome of the review may be accept, revise, or reject.
- Accepted applications may move to Phase 3.
- If revisions are requested, they should be made in a resubmitted application form, with tracked changes and comments, along with a response to the changes in a separate document.
- Rejected applications will be provided with a final determination and reason for the decision.
5. Work with the Add Health Studies Coordinator to develop a cost estimate. The Ancillary Investigator must cover all costs incurred by the study such as: sample selection; collecting or pulling samples from archive; processing and shipping biospecimens; preparing and documenting analysis files; integrating ancillary data into the Add Health Study; and archiving leftover biospecimens. Some of these activities can only be performed by Add Health and/or the Add Health archive lab, which must be paid for by the Ancillary Study.
6. It is Add Health’s expectation that ancillary studies make resulting data available to the Add Health community of data users. Ancillary study approval indicates Add Health’s agreement to work with study investigators to complete the proposed project, conditional on investigators securing the necessary funding to support the work. Approval does not indicate that investigators have the exclusive right to pursue proposed activities. In some instances, ancillary studies may be proposed and approved that overlap in their planned work. In such instances, Add Health will work with investigators to facilitate collaboration.
Phase 3 – Obtain funding and complete documentation
Accepted applications will receive a formal written notice, documenting Add Health’s support for the project and guarantee for collaboration. This notice should be included in any external grant application to fund the project.
Once funding has been received, the Ancillary Investigator will be required to complete the following agreements:
- Data and Material Use Agreement.
- If you do not already have a contract in place, apply for a Restricted-Use Contract through the CPC Data Portal.
- Proof of completion of research ethics training by all research team members who will
work with the Add Health data or biospecimens. - Proof of completion of HIPAA training by all research team members who will work with
the Add Health data or biospecimens (if applicable). - IRB approval for the ancillary study.
- Any changes in the scope or procedures of the study must be submitted via a modification form, reviewed, and approved by Add Health.
Phase 4 – Conducting your study
- Develop a pull list: The Ancillary Study Investigator and Add Health work together to create a participant biospecimens pull list based upon approved selection criteria and Add Health standard biospecimen volumes. Add Health securely and solely maintains the crosswalk from the biospecimen and lab/vial ID to the participant and non-participant IDs.
- Send the biospecimens to Ancillary Study lab: Add Health will work with the storage lab to send the biospecimens to the ancillary study lab and sends a manifest to both Add Health and the ancillary study lab.
- Testing and quality control: The ancillary study lab performs testing. When finished, the ancillary study lab sends results to Add Health linked by a lab/vial ID as well as QC data and internal quality documentation.
- Evaluate data quality: Add Health assesses data quality based upon the participant, non-participant, and IIV biospecimens.
- Receive preliminary dataset: Add Health makes the participant data with masked IDs and internal quality control documentation available to ancillary study investigators to assess the data quality and look for errors.
- Prepare documentation – The Add Health Studies Coordinator will provide a User Guide template for the dataset that the Ancillary Study Investigator will need to create.
- Pay invoice: The Add Health Studies Coordinator will submit an invoice annually for the work completed during the year. Payment must be received before data can be released.
Phase 5 – Release of the Data
Upon satisfying the ancillary study protocols, Add Health exchanges the preliminary IDs for AIDs, then disseminates the AID-identified participant data to the Ancillary Study Investigator. At this time, if the ancillary study is funded by an NIH grant, the Ancillary Investigator is provided exclusive access to the data through the end of the performance period, during which they may submit manuscripts, abstracts, or presentations related to the Ancillary Study. If not funded by NIH, Add Health will adhere to the researcher’s funding agency’s data sharing policies.
At the end of the researcher’s exclusive data access, Add Health will release the final dataset to the user community.