Applying for a Biospecimen Ancillary Study
Add Health maintains a repository of biospecimens that were collected at various timepoints during the study that are available for current use in research. For more information about the use and storage of biospecimens in Add Health, please review the Biospecimen Reserve Policy.
All biospecimens and any data resulting from biospecimen analysis are the property of Add Health and will be made available to the research community according to the terms of the Data and Material Use Agreement. All data sharing between investigators and laboratories must be done by Add Health. It is Add Health policy that data resulting from ancillary studies are made available to the Add Health community of data users. Add Health will comply with the data sharing requirements of the funding agency that supports the work, including the funder that supported the original biospecimen collection. All data dissemination is conducted by Add Health. Add Health does not allow for the commercial use of its data and will not approve ancillary studies that are subject to consulting or licensing obligations to another institution, corporation, or business entity.
Phase 1 – Planning
To gain a more detailed understanding of the application and study process, please refer to the Biospecimen Ancillary Study Researcher Guidelines. Before applying, Biospecimen Ancillary Study investigators should:
- Contact the Ancillary Studies Coordinator to ask any questions.
- Review completed and in progress Ancillary Studies.
- Determine whether the assay(s) can be conducted in Add Health’s partner laboratory or will need to be performed by another laboratory. Add Health recommends that testing be conducted at the Laboratory for Clinical Biochemistry Research (LCBR), our partner laboratory at the University of Vermont (UVM). Our Add Health Biology Team has a longstanding working relationship with LCBR, all of our venous blood biospecimens are archived there, and we can best implement all aspects of your biospecimen testing at this laboratory. If an ancillary study requires a specialized laboratory outside of LCBR, investigators must provide justification for this request that includes plans for final disposition of any leftover sample.
- Determine the minimum possible biospecimen volumes needed to complete the proposed work and only request those minimum volumes. Plan to only run samples in singleton.
- Determine sample size. As part of our quality control protocol, we require the inclusion of internal non-participant laboratory controls and participant intra-individual variation (IIV) replicates. Add Health recommends including non-participant laboratory controls totaling a recommended 5% of the participant sample size. Any proposed reduction in the number of quality control biospecimens must be justified in detail at the time of the ancillary study proposal. Add Health will also include approximately 100 IIV samples depending on the type and sample size of each ancillary study.
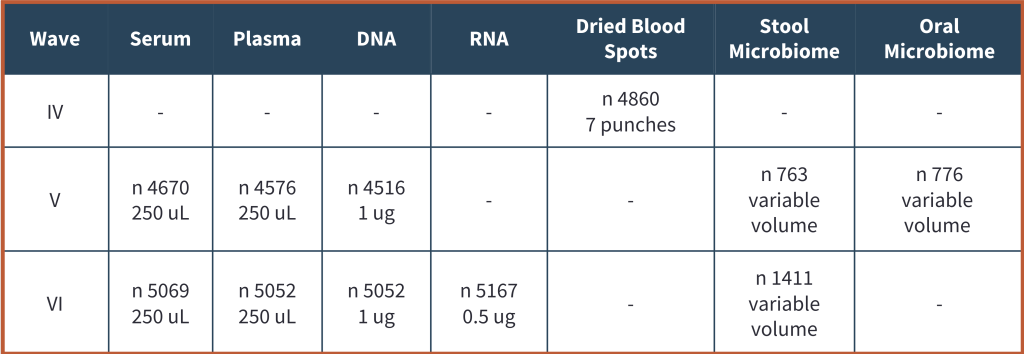
Biospecimen Availability
Phase 2 – Application
Ancillary study applications are reviewed in three steps:
Step 1 – Submit a 1-page concept proposal and preliminary lab form
Concept proposals are reviewed by the ancillary study review committee chair and our central laboratory to determine the feasibility of the study. Applicants are provided feedback and, if approved to proceed, invited to submit a full application.
Step 2 – Submit a budget form
Once the concept proposal is approved, you will need to submit a budget form in order for the Ancillary Studies Coordinator to develop a cost estimate. When preparing the budget, investigators should confirm and include the current cost for preparation and shipping of samples to the testing laboratory being used if other than LCBR. Budget should be included with application submission.
Step 3 – Submit an ancillary study application
After your cost estimate has been prepared, you will be invited to submit a full application that describes the motivation for conducting the project in Add Health, scientific motivation and aims, approach, and timeline. Full applications are reviewed by at least two members of the ancillary studies review committee and our central laboratory. For further guidance on ancillary study review, refer to the review page.
Outcomes of the review process may be:
- Accept – accepted applications will be provided a letter of approval for their grant application. Investigators should also finalize the budget with the Ancillary Studies Coordinator. Note that ancillary study approval indicates Add Health’s agreement to work with study investigators to complete the proposed project, conditional on investigators securing the necessary funding to support the work. Approval does not indicate that investigators have the exclusive right to pursue proposed activities. In some instances, ancillary studies may be proposed and approved that overlap in their planned work. In such instances, Add Health will work with investigators to facilitate collaboration.
- Revise – if revisions are requested, they should be made in a resubmitted application, with tracked changes and comments, along with a response to the changes in a separate document.
- Reject – rejected applications will be provided with a final determination and reason for the decision.
Phase 3 – Funding
Accepted applications will receive a formal written notice documenting Add Health’s support for the project and guarantee for collaboration. This notice should be included in any external grant application to fund the project.
After receiving funding, the Ancillary Study Investigator will be required to complete the following distribution agreements prior to the release of any data by the Carolina Population Center (CPC)/University of North Carolina.
- Review, fill and sign the Data and Material Use Agreement.
- Apply for a Restricted-Use Contract if one is not already in place by going to the CPC Data Portal.
- Provide proof of completion of research ethics training by all research team members who will work with the Add Health data.
- Provide IRB approval for the ancillary study.
Annual Status Reports and Invoicing
After an Ancillary Study is funded and initiated, the PI is responsible for submitting annual progress reports of the study’s status to Add Health until Add Health has released final ancillary data. These progress reports must summarize the study’s activities, including:
- Progress of data collected, biospecimens received and lab testing to date
- Analysis in progress or completed
- Updates to timeline
Annual report forms will be sent out to investigators no later than June 1 of every year in the study period. These forms must be completed and returned to CPC no later than July 1 of the same year. Ancillary studies that fail to comply with the annual reporting requirement may be ineligible for renewal of their Add Health restricted-use contract and/or their Data and Material Use Agreement.
The Add Health Studies Coordinator will submit an invoice annually for the work completed during the year. Payment must be received before any data can be released.
Phase 4 – Conduct your study
As you conduct your study, it is important to communicate regularly with the Add Health team. The Add Health Ancillary Studies Coordinator schedules a kick-off meeting to discuss your study, review the ancillary process and your timeline, and answer any questions.
Study Modifications
After an Ancillary Study is approved, changes in the scope or procedures of the study must be submitted via a modification form, and must be reviewed, and approved by Add Health. Any post hoc changes to approved ancillary study selection criteria, sample size, or protocol must be formally proposed and justified in a modification request submitted to the Ancillary Studies group. Formal review and approval of such requests must precede sample selection. Immediately report any unanticipated changes in laboratory protocol, reagent shortages, substituted materials, or adverse events to the Ancillary Studies Coordinator.
Key Steps
To ensure your study runs smoothly and maintains the highest quality standards, it is essential that the Add Health Ancillary Studies Coordinator is included in your communications with the lab. This inclusion helps us stay informed about the study’s progress and prevents any issues with biospecimen tracking or data flow between the lab and your institution. Below is a list of key junctures in which you should speak with the Add Health staff.
Step 1 – Develop a pull list: The Investigator and Add Health work together to create a participant biospecimen pull list based upon approved selection criteria, sample size, and volume.
Step 2 – Masking IDs: Add Health will replace participant IDs with undifferentiated, masked ancillary study IDs before shipping samples to laboratories for assay. Add Health securely and solely maintains the crosswalk between participant IDs and ancillary study IDs.
Step 3 – Send the biospecimens to Ancillary Study lab: Add Health works with the appropriate repository location to send the biospecimens to the laboratory approved for the ancillary study along with a manifest.
Step 4 – Testing and quality control: The laboratory performs testing. When finished, the laboratory sends results to Add Health along with QC data and internal quality control documentation. Internal quality control documentation should include a summary of procedures and assay data quality (e.g., counts of missing values, differences in correlations between known and assayed values, coefficients of variation within/between split samples, identification of masked duplicate pairs for studies requesting DNA).
Step 5 – External Quality Control: Add Health assesses data quality based upon the participant, nonparticipant, and intra-individual variation biospecimens (described in Planning). Add Health will produce an external quality control report that includes standard reliability and validity measures and summary statistics for high-dimensional data.
Step 6 – Receive preliminary dataset: Add Health makes preliminary ancillary study data with temporary ancillary study IDs and quality control documentation available to investigators to assess the data quality and look for errors.
Step 7 – Prepare documentation: The Ancillary Studies Coordinator will provide a User Guide template that investigators will use to prepare the required documentation for the ancillary study data.
Step 8 – Pay invoice: The Ancillary Studies Coordinator will submit an invoice annually for the work completed during the year, and at the end of the work completed. Payment must be received before any data can be released.
Step 9 – Return biospecimens: If applicable, include a return inventory log showing remaining, post-assay biospecimen type and volume and include this electronic document alongside all returned leftover biospecimens to the Add Health biorepository.
Phase 5 – Receive your data
Upon satisfying the ancillary study protocols, Add Health exchanges the ancillary study IDs for Add Health participant IDS (AIDs), then disseminates the AID-identified participant data to the Ancillary Study Investigator. In order to receive ancillary study data, the investigator will need to apply for a restricted-use data contract. This contract is an agreement signed by two parties, the Ancillary Study Institution and UNC-Chapel Hill on behalf of Add Health. To apply for restricted-use data, please use the Add Health Contracts Management System (CMS) via the CPC Data Portal. There are two types of restricted-use contract options – Secure Research Workspace (SRW) or Home Institution – dependent on your current or intended method of accessing Add Health restricted-use data.
Add Health follows the data sharing policies of the National Institutes of Health that supported the collection of the biospecimens. Under the 2023 Data Management and Sharing Policy, scientific data should be made accessible as soon as possible. Data should be shared by the time of the first associated publication or the end of the performance period, whichever comes first.
Data Security
Protecting the identity of individual Add Health respondents is a critical issue for the Add Health study. Confidentiality of individually identifiable data about Add Health participants must be assured. Unless the approved restricted-use data contract allows otherwise, all data work will take place in the Secure Research Workspace (SRW). The SRW helps keep Add Health data more secure in our efforts to maintain the strict confidentiality of our participants. The SRW is available through remote access. All research and analysis will take place only within the SRW.