Applying for a Contextual Ancillary Study
To gain a more detailed understanding of the application and study process, please refer to the Contextual Ancillary Study Researcher Guidelines. Conducting a contextual ancillary study involves five phases:
Phase 1 – Planning
Before applying, investigators should:
- Contact the Ancillary Studies Coordinator to ask any questions.
- Review previously conducted and approved Ancillary Studies to ensure there is no significant overlap in concepts.
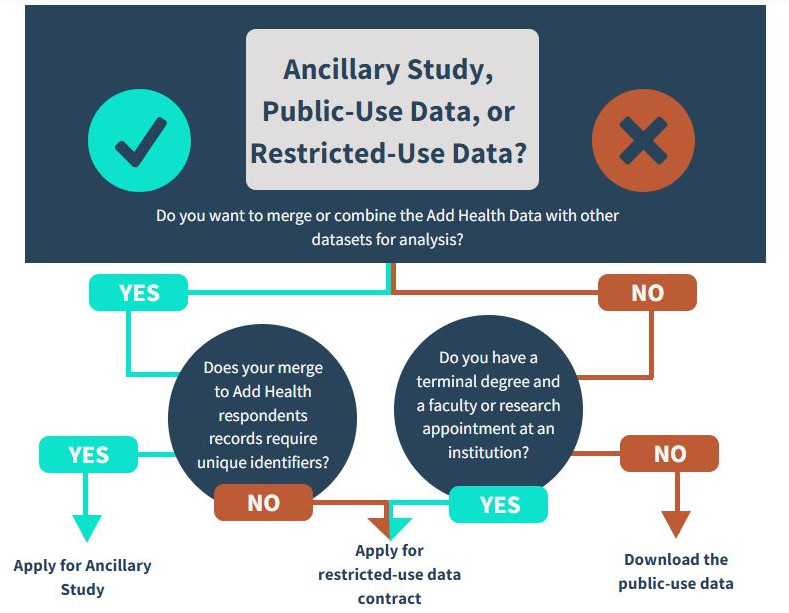
- Determine if you require an ancillary study or if your research can be conducted with a restricted use data contract. Please see the chart below for assistance.
- Review the Add Health codebooks for existing datasets to confirm data do not currently exist.
- Add Health Ancillary Study approval should be obtained before any grants including the planned activities are submitted.
Phase 2 – Applying
Step 1. Submit a 1-page concept proposal – Concept proposals are reviewed by the ancillary study review committee chair to determine the feasibility of the study. Applicants are provided feedback and, if approved to proceed, invited to submit a full application. Proposals can be submitted to the Ancillary Studies Coordinator. Examples are available under the Documents and Resources tab.
Step 2. Submit an ancillary application – Your full application should describe the motivation for conducting the project in Add Health, scientific motivation and aims, approach, and timeline. Full applications are reviewed by at least two members of the ancillary studies review committee. For further guidance on ancillary study review, refer to the review page. The Ancillary Investigator must cover all costs incurred by the study such as preparing and documenting analysis files, data merge, codebook creation, and integrating ancillary data into the Add Health Study. Please work with the Ancillary Studies Coordinator to develop a cost estimate.
Outcomes of the review process may be:
- Accept – accepted applications will be provided a letter of approval for their grant application. Investigators should also finalize the budget with the Ancillary Studies Coordinator. Note that ancillary study approval indicates Add Healthʼs agreement to work with study investigators to complete the proposed project, conditional on investigators securing the necessary funding to support the work. Approval does not indicate that investigators have the exclusive right to pursue proposed activities. In some instances, ancillary studies may be proposed and approved that overlap in their planned work. In such instances, Add Health will work with investigators to facilitate collaboration.
- Revise – if revisions are requested, they should be made in a resubmitted application, with tracked changes and comments, along with a response to the changes in a separate document.
- Reject – rejected applications will be provided with a final determination and reason for the decision.
Phase 3 – Funding and Agreements
Accepted applications will receive a formal written notice documenting Add Healthʼs support for the project and guarantee for collaboration. This notice should be included in any external grant application to fund the project.
After receiving funding, the Ancillary Study Investigator will be required to complete the following distribution agreements prior to the release of any data by the Carolina Population Center (CPC)/University of North Carolina.
- Review, fill, and sign a Data and Material Use Agreement.
- Apply for a Restricted-Use Contract if one is not already in place by going to the CPC Data Portal.
- Provide proof of completion of research ethics training by all research team members who work with the Add Health data.
- Provide IRB approval for the ancillary study.
Annual Status Reports and Invoicing
After an Ancillary Study is funded and initiated, the PI is responsible for submitting annual progress reports of the study’s status to Add Health until Add Health has released final ancillary data. These progress reports must summarize the study’s activities, including:
- Progress of data collected to date
- Analysis in progress or completed
- Updates to scope, design, or methods
- Updates to timeline
Annual report forms will be sent out to investigators no later than June 1 of every year in the study period. These forms must be completed and returned to CPC no later than July 1 of the same year. Ancillary studies that fail to comply with the annual reporting requirement may be ineligible for renewal of their Add Health restricted-use contract and/or their Data and Material Use Agreement.
Invoices are submitted after the data has been merged and must be paid before the data can be released.
Phase 4 – Conduct your study
Step 1: Schedule your launch meeting – Once your required documents are submitted and funding has been obtained, the Add Health Studies Coordinator will schedule a meeting with the Ancillary Study Investigatorʼs team and Add Health to discuss your data plans and answer any questions.
Step 2: Data set preparation – After data collection is completed, it is time to assemble the planned contextual variables. Be sure to document data sources, variable construction and other relevant details. This information should include, but not be limited to:
- a detailed description of the source data.
- programming code documenting any variable construction.
- references for variable constructs (e.g., the reference for the standard scale used).
- documentation on the cleaning process to date.
Step 3: Submit clean data file for merge – Once Add Health has received the files, staff will examine the data for deductive disclosure risks and modify the data to reduce these risks. During this time, the following will be conducted:
- Ancillary data will be merged with existing Add Health data.
- Variable construction of the added data will be tested, researched, and reviewed. Consultants may be contacted, as necessary.
- Frequency distributions of the added variables will be evaluated for deductive disclosure.
- Cross tabulations of the new data with existing Add Health data will be run and reviewed.
- Logical associations among the new and existing data will be mapped out and evaluated.
- Documentation will be examined and edited.
- Decisions about modifications to the variables will be made in consultation with the Ancillary Studies Investigator. This may involve dropping variables, collapsing categories, or modifying the data in other ways to protect the identity of the study participants.
- A preliminary data file will be created.
- Codebooks following the Add Health standards will be created.
Step 4: Receive preliminary dataset – After the data is merged, the preliminary with masked participant IDs dataset will be placed on the secure research workspace (SRW) and the Ancillary Study Investigator will be given 30 days to review the data for errors. If an error is found, Add Health will make the necessary corrections and the Ancillary Study Investigator will be given an additional 30 days to review.
Once the data has been approved by the Ancillary Study Investigator, the preliminary dataset will be removed from the SRW and Add Health staff will begin preparations to release the final datasets. Because the final dataset is likely to be different from this preliminary dataset, the investigator is encouraged to develop & document analysis programs so they may be re-run against the final dataset that is released to the public.
Step 5: Prepare Documentation – The Add Health Studies Coordinator will provide a User Guide template for the dataset that the
Ancillary Study Investigator will need to create.
Step 6: Pay invoice – After the preliminary data has been approved, Add Health will conduct a final disclosure review and
prepare the data for dissemination. The Ancillary Studies Coordinator will submit an invoice at this time for payment. Payment must be received before data can be released.
Phase 5 – Release of the data
Once approval to disseminate is received from the Ancillary Study Investigator, a tentative release date will be set for the new data. The timeframe for release will be determined by the number of variables included in the ancillary dataset and the availability of Add Health staff to work on the final deductive disclosure risk review. The final data will be released to the entire user community at the same time. The Ancillary Study Investigator can request the data via the CPC Data Portal and begin publishing and presenting on the data.
The determination of what is included in the released file is made in collaboration with the Ancillary Study Investigator and their team. The Add Health Director/PI may assist in making decisions if needed.
Ancillary Study Investigators are not permitted to submit any manuscripts, abstracts, or presentations derived from the Ancillary Study for review before the release date of the final file. The Ancillary Study Investigator agrees to use only data from the final released file for analysis and submitted manuscripts, and any such work must include appropriate attribution to Add Health as specified in the Add Health restricted-use data agreement.